Guest Post by Dr. R. Bruce Prime
In the previous post we introduced modulated temperature DSC and described the basic principles. Here we show how MTDSC can be used to provide insight into the nonisothermal cure of thermosets, much of which cannot be obtained by standard DSC.
The figure below shows the MTDSC of an uncured amine-epoxy system at 1 ˚C/min (±1°C/60s). In Part a, the nonreversing heat flow or NHF, we see what appears to be a normal cure exotherm. But upon close inspection the return to the baseline near the completion of cure can be seen to slow, starting about 140 ˚C. Part b compares the sample temperature (= cure temperature Tcure, dashed line) with the glass transition temperature (solid line, computed from modeling) during cure. Remember that Tg increases as a result of the cure reaction. Note that the lines cross three times at Tg = Tcure where either a vitrification or devitrification transition occurs {see Fig. 105 and pp 1588-1597 in Thermal Characterization of Polymeric Materials (E. A. Turi, ed.), (1997)}. Part c shows the reversing heat capacity (Cpr) for the first heat (uncured epoxy) and second heat (fully cured epoxy). The first heat is rich in information, starting with a step increase in heat capacity at the devitrification of the uncured epoxy at Tg0 = -16 ˚C, where the lines in Part b first cross. Following that Tg remains constant until it starts to rise with the onset of the cure exotherm at about 50 ˚C. Note in Part b that during the most rapid part of cure Tg rises faster than Tcure, the lines cross for the second time and vitrification occurs as indicated by the step decrease in heat capacity near 140 ˚C. Cure continues in the glassy state until the reaction is complete and devitrification of the fully cured epoxy occurs at Tg¥ where the lines cross for the third time.
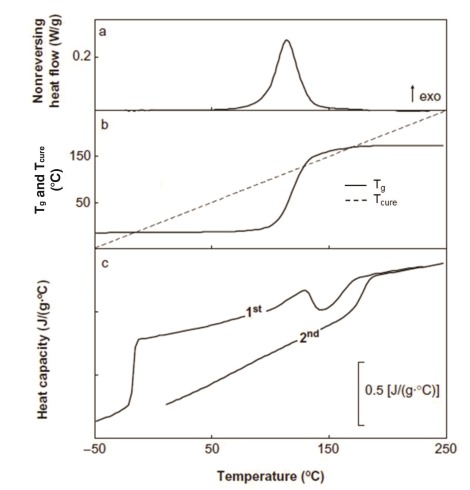 Diglycidyl ether of bisphenol-A (DGEBA) / methylene dianiline (MDA) system with 1:1 stoichiometry. From Swier et al. J. Appl. Polym. Sci. 91, 2814 (2004).
Diglycidyl ether of bisphenol-A (DGEBA) / methylene dianiline (MDA) system with 1:1 stoichiometry. From Swier et al. J. Appl. Polym. Sci. 91, 2814 (2004).
Curing at heating rates that are slow enough to maintain the thermoset in the glassy state but still fast enough to complete the cure in a reasonable amount of time has practical applications. For example, it can be employed to control the exotherm of highly reactive systems and prevent thermal runaways, especially dangerous when large masses are involved. And it can be used to complete cure without expensive fixturing while maintaining dimensional stability. One such application shown below is the amplifier used in the Bell System undersea cable {H. E. Bair and S. Matsuoka, US Patent 4,978,712 (1990)}. The optical fiber is held in alignment and partially cured to Tg » 60 ˚C (Tg¥ = 105 ˚C). Then the device is removed from the fixture and the cure of several devices are completed by heating them in an oven at about 0.5 ˚C/min until cure is complete, which satisfies the above criteria.
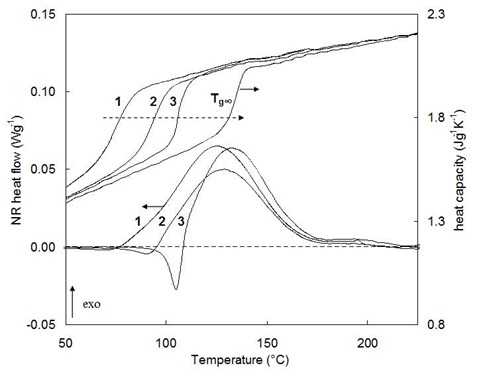
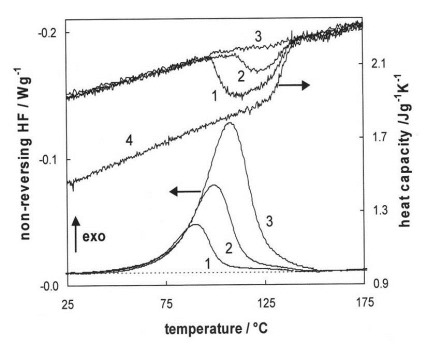
We can now interpret the MTDSC figure below, which shows the NHF and Cpr curves for an epoxy system at three ramp rates. Curve 4 is the Cpr after full cure which shows the glassy heat capacity and Tg¥ at about 140 °C.
-
At 0.2 °C/min ramp rate (1) we see the liquid/rubbery heat capacity begin to decrease just below 100°C upon vitrification and remain between the liquid/rubbery and glassy heat capacities as cure proceeds in the partially vitrified state. When cure is complete the heat capacity returns to the liquid/rubbery line.
-
At 0.4 °C/min (2) vitrification occurs at a higher temperature and to a lesser extent.
-
At 0.7 °C/min (3) vitrification is not observed and cure is accomplished entirely under chemical control.
Thus MTDSC tells us that a heating rate around 0.4 °C/min would be appropriate to complete the cure of this system in the glassy state. Measurements such as these will save valuable time compared to the alternative trial and error methods. They are also useful for determining the minimum heating rate for multiple heating rate experiments where cure needs to proceed without vitrification.
Non-isothermal cure of an epoxy-anhydride. From Van Mele et al. Chapter 2 in Modulated-Temperature Differential Scanning Calorimetry: Theoretical and Practical Applications in Polymer Characterisation, Reading and Hourston eds., Springer, Vol. 6 (2006).
The figure below shows the NHF and Cpr for an epoxy-anhydride system cured to different degrees at Tcure = 85 °C. After full cure we observe that Tg¥ for this system is 135°C. Curve 1, after 165 min at 85 °C, shows a Tg that is below Tcure and well separated from the residual exotherm without significant enthalpy relaxation, all indicating that vitrification had not occurred during the isothermal cure. After 230 min we see in Curve 2 Tg in the vicinity of Tcure accompanied by a small enthalpy relaxation peak. After 800 min we can observe in Curve 3 that Tg has increased 15-20 °C beyond Tcure and is accompanied by a large enthalpy relaxation peak indicating that vitrification has occurred plus significant physical aging. Note the overlap of the enthalpy relaxation endotherm with the residual cure exotherm.
 MTDSC of Epoxy Anhydride after Partial Cure at 85 °C. 2.5C °/min (±1 °C/60s). From Van Mele et al. Chapter 2 in Modulated-Temperature Differential Scanning Calorimetry: Theoretical and Practical Applications in Polymer Characterisation, Reading and Hourston eds., Springer, Vol. 6 (2006).
MTDSC of Epoxy Anhydride after Partial Cure at 85 °C. 2.5C °/min (±1 °C/60s). From Van Mele et al. Chapter 2 in Modulated-Temperature Differential Scanning Calorimetry: Theoretical and Practical Applications in Polymer Characterisation, Reading and Hourston eds., Springer, Vol. 6 (2006).
To review, MTDSC can identify appropriate heating rates for curing in the glassy state to maintain dimensional stability, as well as the minimum heating rate for multiple heating rate experiments in order to avoid vitrification. On partially cured samples MTDSC gives clean measurements of Tg and the residual cure exotherm for kinetic studies. In the next post we explore the application of MTDSC to isothermal cure.
Dr. Lawrence Judovits is acknowledged for his contribution to this post.

Leave a Reply