Over the last several posts we have been discussing how fillers can be used to significantly modify the physical properties of thermosets including lowering the coefficient of thermal expansion (CTE) and increasing the thermal conductivity. In this post we will discuss some ways fillers can be used to make electrically conductive thermosets. A good example of an electrically conductive, highly filled thermoset is a die attach adhesive. Many die attach adhesives provide an electrical path between the semiconductor chip (die) and the substrate, typically a metal leadframe. In the image on the left, the die attach (purple) is shown between the die and the die pad in a quad flat pack no lead (QFN) package.
As we discussed in an earlier post, unfilled polymers are good insulators, both thermally and electrically. Many polymers are used explicitly to take advantage of the electrical insulation property:
- cable and wire insulation
- electrical terminal strips
- electrical boxes
- dielectric layers between circuits in semiconductor substrates and circuit boards
- underfills used to insulate between solder bumps or solder pillars
- many more
In order to achieve electrical conductivity, fillers are required. In this case, metal fillers, specifically silver flakes are used extensively. An added benefit of using silver (or other metal fillers) is that no only can you increase the electrical conductivity, but the thermal conductivity is also increased. Remember our post on increasing thermal conductivity and we mentioned that the filler aspect ratio is important. For high electrical conductivity (or lower resistivity, you see both types of values on technical data sheets) using a plate-like filler is essential. We talked about stacking “poker chips” versus “marbles.” In Figure 1, an SEM image shows the morphology of silver flakes. The silver flake example is AA-0707 from Metalor ( click to visit Metalor silver flake product catalog).
Figure 1. SEM image of Metalor AA-0707 (courtesy of Metalor)
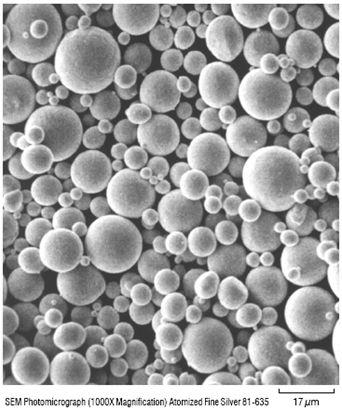
In Figure 1, the plate-like morphology is clearly visible. Silver flakes can be obtained in a wide range of particle sizes and morphologies. Contrast the morphology in Figure 1 with the SEM image of Technic fine silver powder 81-635 shown in Figure 2.
Figure 2. SEM image of atomized silver powder 81-635 (courtesy of Technic, Inc.)
There are two key features of fillers used in electrical conductive compositions. The first is the morphology and the second is the filler surface condition. The morphology is important to get very high particle-to-particle interactions thus allowing good electron flow. Silver flakes are almost used exclusively for electrical conductive thermoset compositions. The flake geometry allows for large surface area flakes to easily come in contact with other flakes. One consideration that must be taken into account is that silver has very high attraction for silver, which is good for developing high electrical conductivity, but must be managed to allow for the flakes to be mixed into the thermoset formulation. Silver flakes are produced by milling silver powders in the presence of a lubricant. The lubricant (typically fatty acids) coat the surface of the flake and prevent the flakes from sticking together allowing good mixing into a low viscosity thermoset formulation. The silver flake vendors provide a wide range of lubricants depending on the application and the chemistry of the formulation. Additionally, silver flake may be obtained over a wide particle size range. Depending on the dispensing or application method, the choice of the right silver flake (average flake size) and lubricant chemistry is critical in order to achieve very low electrical resistance after curing.



Leave a Reply