Guest Post by Dr. Robert Humphreys.
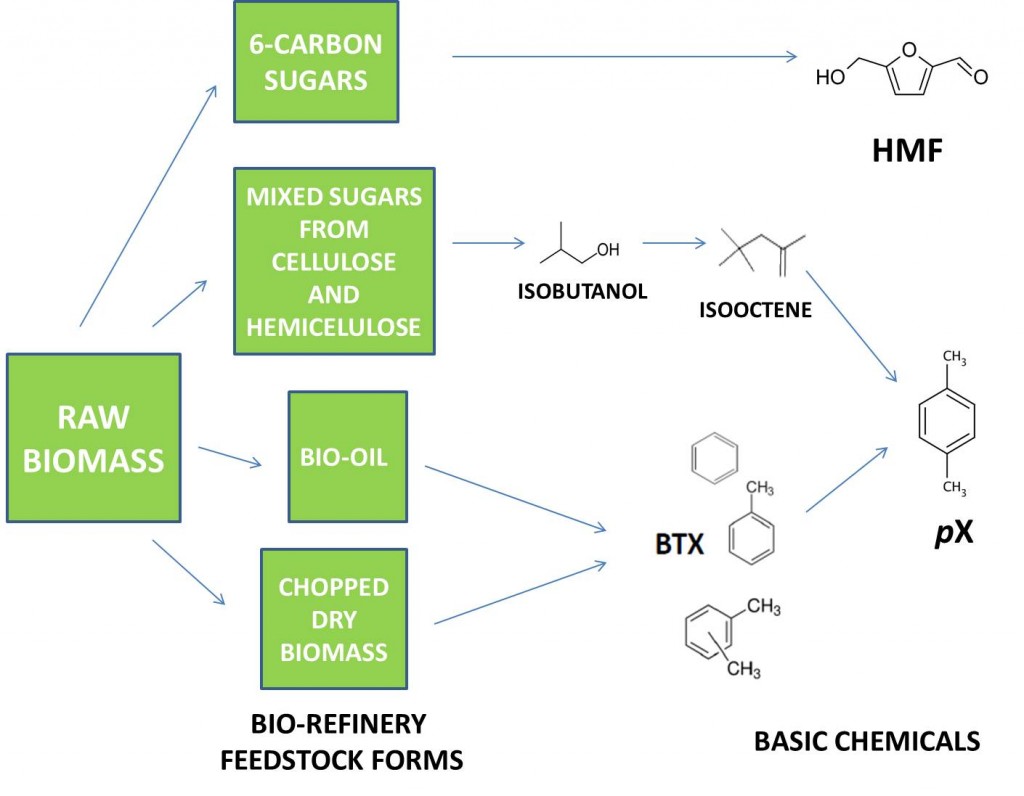
 In several recent posts, we discussed the composition of biomass and how crude biomass can be converted into forms of feedstock that can be handled easily in a bio-refinery. We outlined processes for making three feedstock forms: concentrated sugars from hydrolysis of the cellulose and hemicellulose components of biomass; bio-oil from fast pyrolysis of biomass; and dry, finely chopped biomass. Each of these feedstock forms can be converted to basic chemicals. Remembering our commitment to describe the conversion of biomass to renewable beverage bottles, we will now focus on how these three feedstock forms can be converted into basic chemicals that can be used to make monomers for bio-plastic beverage bottles.
In several recent posts, we discussed the composition of biomass and how crude biomass can be converted into forms of feedstock that can be handled easily in a bio-refinery. We outlined processes for making three feedstock forms: concentrated sugars from hydrolysis of the cellulose and hemicellulose components of biomass; bio-oil from fast pyrolysis of biomass; and dry, finely chopped biomass. Each of these feedstock forms can be converted to basic chemicals. Remembering our commitment to describe the conversion of biomass to renewable beverage bottles, we will now focus on how these three feedstock forms can be converted into basic chemicals that can be used to make monomers for bio-plastic beverage bottles.
From Biomass to pX
Most plastic beverage bottles are made from polyethylene terephthalate (PET), a copolymer of ethylene glycol (EG) and terephthalic acid (TA). Successful replacement of petro-based PET with a “drop-in equivalent,” renewable version requires economical, renewable sources for both monomers. Bio-EG is produced commercially from ethanol, so attention has shifted to developing renewable sources of p-xylene (pX), the basic petrochemical used to make approximately 28 million metric tons of TA per annum globally. Processes that are being explored for conversion of all three forms of biomass feedstock to pX are outlined below.
1) Fermentation of sugars followed by chemical conversion to pX
Fermentation is one of the metabolic processes by which organisms such as bacteria or yeast convert a food source, such as simple sugars, into chemical energy that is used to carry out all of the other processes of life. Synthetic biologists have learned how to redirect the metabolism of bacteria and yeast so that much of the chemical energy from fermentation is used to convert sugar into molecules that can be used as fuel or chemicals instead of simply producing more cells. A good example of a molecule that can be produced by fermentation of sugars is isobutanol, a 4-carbon molecule that can be dehydrated and dimerized to the 8-carbon molecule, isooctene. Isooctene can be converted directly to pX, which is also an 8-carbon molecule. The process depends on special catalysts. Isobutanol can be converted to fuels and other chemicals, too, which enhances its attractiveness as a fermentation target.
2) Catalytic conversion of sugars and bio-oil to pX
Sugars or bio-oil can be converted to pX using appropriate catalysts and heat in multi-step processes. Both sugars and bio-oil contain a high level of oxygen, particularly compared to hydrocarbons such as pX. Therefore, conversion of glucose or bio-oil to pX requires replacement of the oxygen with hydrogen. In fermentation of sugars, the organism supplies the hydrogen by converting some of the sugar to carbon dioxide. In catalytic conversion of sugar, bio-oil, or biomass, the hydrogen can be generated catalytically from petroleum or by sacrificing some biomass to produce hydrogen and carbon dioxide. Catalytic conversion of sugars or bio-oil produces mixtures of products, including benzene, toluene, and xylene (BTX), from which pX can be separated. Technology to isomerize the mixture and, thereby, enhance the yield of xylene also can be employed. These processes can be adjusted to produce fuels and other chemicals.
3) Conversion of dry, chopped biomass to pX
Biomass can be converted thermally to mixtures of products without prior conversion to sugars or bio-oil. Particulate biomass is pyrolized (heated to high temperature) in a reactor containing a catalyst. The pyrolysis products undergo further reaction downstream in the presence of other catalysts chosen to favor a product mixture that is rich in aromatic molecules including benzene, toluene, and xylene (BTX), from which pX can be separated. Again, isomerization can be employed to enhance the yield of pX and process conditions can be varied to produce fuels and other chemicals.
From Biomass to HMF
In a previous post, we mentioned polyethylene furanoate, or PEF, a 100% renewable polymer with properties that make it a potential alternative to PET for renewable plastic beverage bottles. PEF is a copolymer of bio-EG, a commercially available monomer, and furan-1,5-dicarboxylic acid (FDCA), a monomer that can be made from sugar and is not available commercially on a large scale. Like TA, FDCA is produced from a precursor, in this case 5-hydroxymethylfurfural (HMF). HMF is the direct product of acid-catalyzed rearrangement of 6-carbon sugars such as glucose and fructose. Thus, cane sugar and cellulose-derived glucose are potential raw materials for HMF and FDCA.
And the winning technology is…..?
It should be clear from the information above that there are many options for converting biomass to renewable, basic chemicals that can be used to produce bio-plastics. All technology options will require a huge, dependable supply of non-food biomass in a process friendly form. Which technology will win in the marketplace is far from clear and will depend on a host of factors that face any new technology directed at a market as massive as plastics. This may explain why major users of packaging plastics such as beverage and consumer products companies are hedging their bets by backing multiple technologies. We have summarized the various technologies in the accompanying graphic and we note that the space limitations have necessitated much simplification of the various processes.
The Final Step
We aren’t finished yet. HMF and pX must still be converted to FDCA and PTA before renewable beverage bottle plastics can be made. In the final post in this series, we will cover how this transformation occurs and how these monomers are combined with bio-EG to make renewable plastics.

Leave a Reply