Guest Post by Dr. Robert Humphreys
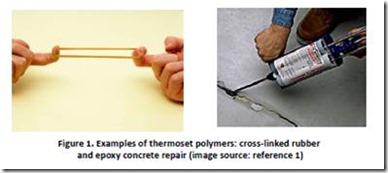
 Thermoset polymer systems undergo a transformation, often referred to as “curing”, from a liquid or a soft solid in which the molecules can move around freely (i.e. the uncured material will flow) to a material in which molecules are interconnected to form a network throughout the material (the cured material will not flow, even under stress). Before cure, the thermoset can be coated on a surface or shaped by molding or dissolved in a suitable solvent. After cure, the thermoset will not dissolve and resists a change in shape under stress. The curing process involves the formation of new chemical bonds between molecules in the thermoset, resulting in most molecules being linked in a continuous network. This network will resist a change in shape, with the degree of resistance depending on the properties of the linked molecules. For example, cured rubbers will deform under stress but will return to their cured shape when the stress is removed (think of a stretched “rubber” band) while a properly formulated structural epoxy adhesive will cure to form a solid that is very hard and strong enough to repair concrete.
Thermoset polymer systems undergo a transformation, often referred to as “curing”, from a liquid or a soft solid in which the molecules can move around freely (i.e. the uncured material will flow) to a material in which molecules are interconnected to form a network throughout the material (the cured material will not flow, even under stress). Before cure, the thermoset can be coated on a surface or shaped by molding or dissolved in a suitable solvent. After cure, the thermoset will not dissolve and resists a change in shape under stress. The curing process involves the formation of new chemical bonds between molecules in the thermoset, resulting in most molecules being linked in a continuous network. This network will resist a change in shape, with the degree of resistance depending on the properties of the linked molecules. For example, cured rubbers will deform under stress but will return to their cured shape when the stress is removed (think of a stretched “rubber” band) while a properly formulated structural epoxy adhesive will cure to form a solid that is very hard and strong enough to repair concrete.
Drying oils such as linseed and tung oils were the first renewable, thermoset polymers used by mankind. When exposed to air for sufficient time, a film of liquid drying oil “cures” to form a sticky coating that will eventually harden into a film that can be used as a moisture resistant barrier 2. Over several millennia, the technology was improved dramatically to become the dominant form of protective and decorative coating for wood and other surfaces until the mid-20th century, when it was largely supplanted by alkyd resins and water-based coatings made with petroleum-derived polymer technology. Drying oils can also be formulated to make hard, wear resistant materials, as exemplified by the floor covering known as Linoleum that was invented in the 19th century and is still sold today 2b.
The recent explosion of interest and research in chemicals and polymers from renewable raw materials (RRMs) has led to many new approaches to renewable thermoset technology. Some are based on direct utilization of RRMs, such as unsaturated fats and oils, in thermosets; others require fermentation and/or chemical conversion of RRMs such as sugars to monomers, cross-linking agents and polymers for thermosets. We will discuss direct utilization of RRMs in the remainder of this posting, to be followed in the next posting by an overview of thermoset monomers, cross-linking agents and polymers from fermentation and/or chemical processing of RRMs. Where possible, we will focus attention on materials that are commercially available or are being commercialized.
Thermosets Directly from RRMs: Vegetable Oil Derivatives and Natural Rubber
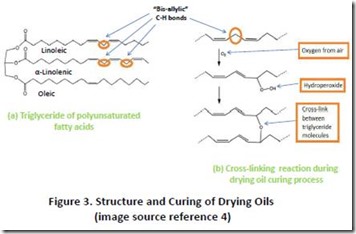
For readers who are familiar with high-performance thermoset materials such as structural epoxy, polyurethane and acrylic adhesives and composites, it may seem strange to refer to coatings based on drying oils as thermosets. Nevertheless, they fit the definition since they undergo chemical cross-linking during the curing process. Drying oils consist of triglycerides containing high levels of polyunsaturated fatty acids, such as linoleic and linolenic acid. Typical polyunsaturated fatty acids contain one (eg. linoleic acid) or two (eg. linolenic acid) “bis-allylic” methylene groups, marked clearly in the red ovals in Figure 34. This bis-allylic position is very susceptible to oxidation by molecular oxygen in air (a process known as “autoxidation”), which leads to formation of hydroperoxides and, ultimately, bond formation between different triglyceride molecules (Figure 3b). A hard film is formed when sufficient cross-linking bond formation has occurred. Metal catalysts can dramatically accelerate the rate of curing. Properly formulated drying oils make excellent protective coatings (paints and stains) and durable flooring.
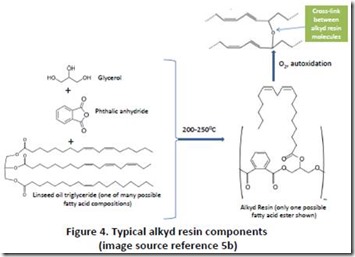
Alkyd resins are related technology, usually formulated as mixtures of renewable and petroleum-derived ingredients. While processes and compositions vary considerably, a typical alkyd resin is prepared by cooking at elevated temperature (>2000C) a mixture of polyunsaturated triglyceride, a polyhydric alcohol (a molecule with three or more hydroxyl groups) and a dicarboxylic acid (usually phthalic acid or anhydride) until a product of the desired viscosity is obtained 5. As with drying oils, alkyd resin films cure in the presence of atmospheric oxygen and can be formulated to make durable paints, varnishes, and coatings for electrical equipment 6. Figure 4 shows the structure of the major components of a typical alkyd resin. At present, phthalic acid is a petroleum-derived raw material, so about 70% of a typical alkyd resin is renewable.
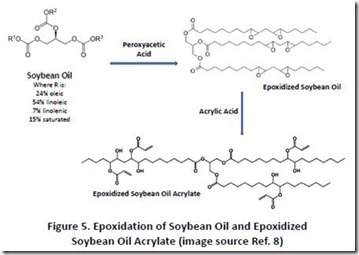
The carbon-carbon double bonds in polyunsaturated natural oils can also be converted into reactive epoxy groups 7 using epoxidizing reagents, such as peroxyacids (see Figure 5 8), hydrogen peroxide or hydroperoxides with catalysts, and even certain enzymes. Academic publications and patents have described both properties and applications of epoxidized natural oils with much of the work focusing on materials from linseed and soybean oils. Epoxidized linseed and soybean oils are available commercially 9 for applications such as coating. Acrylate-functionalized derivatives of epoxidized soybean oils are also available; they are made through addition of acrylic acid to the epoxy groups for applications such as printing inks 10.
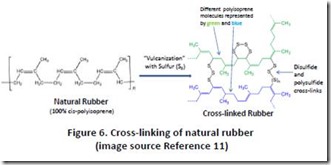
Finally, natural rubber should be included in any discussion of thermosets derived directly from renewable materials. Natural rubber is polyisoprene with 100% of the carbon-carbon double bonds in the cis-configuration (the polymer chain is on opposite sides of the C-C double bond: see Figure 5). The combination of C-C double bonds with allylic C-H bonds makes polyisoprene very susceptible to cross-linking by free radical reactions such as the vulcanization process, which involves a complex mixture of reactants including elemental sulfur (Figure 6 11). With proper formulation, cross-linked natural rubber can form extremely tough, resilient materials, as exemplified by tires.
It is evident that excellent protective coatings can be formulated from natural oils (eg. linseed and soybean oil) and chemically modified natural oils (alkyds and epoxidized oils). Attempts to formulate high performance thermoset materials based on natural oils as the only cross-linking agent have met with less success; although there is ample literature on the subject, the technology has provided few commercial products12. This is not surprising since the cross-link density achievable with natural oils is too low to provide properties necessary for true structural applications. In the next post, we will discuss how renewable materials can be applied to high performance thermoset polymers.
References:
1. Rubber band image from momstownkitchener-waterloo.blogspot, see rubber band image; epoxy concrete repair image from everything-about-concrete.com, see concrete repair .
2. Michael R. Van De Mark and Kathryn Sandefur, “Vegetable Oils in Paint and Coatings”, Inform, August 2005, 16(8), 478-480; b) See, for example, https://www.armstrong.com/flooring/products/linoleum ; https://www.thisoldhouse.com/toh/article/0,,202857,00.html
3. Linoleum floor image from ECO Building Products site, https://eco-buildingproducts.com/product/marmoleum-mct-natural-flooring/ ; Alkyd house paint image from ehow.com site, paint image .
4. Structures in Figure 3 taken from Google, see: https://en.wikipedia.org/wiki/File:DryingOilDiene%27.png
5. a) “Alkyd paints and driers”, see https://openaccess.leidenuniv.nl/bitstream/handle/1887/2309/01.pdf ; b) Figure 4 images from Google (glycerol, phthalic anhydride, and drying oil) and springerimages.com (see Springer images ).
6. Edward N. Peters, “Plastics: Thermoplastics, Thermosets, and Elastomers”, see https://onlinelibrary.wiley.com
7. a) Li Shen, Juliane Haufe, and Martin K. Patel, “Product overview and market projection of emerging bio-based plastics, PRO-BIP 2009”, June 2009, available at reference 7; b) S. Tayde, M. Patnaik, S. L. Bhagt, and V.C. Renge, “Epoxidation of Vegetable Oils: A Review”, International Journal of advanced Engineering Technology, 2001, 2, 491-501; c) J.-M. Raquez, M. Deleglise, M.-F. Lacrampe, and P. Krawczak, “Thermosetting (bio)materials derived from renewable resources: A critical review”, Progress in Polymer Science, 2010, 35, 487-509; d) Rafael Lopes Quirino, “Natural Oil-based Thermosets and Composites”, https://www.biocom.iastate.edu/workshop/2012workshop/presentations/quirino.pdf
8. Figure 5 images taken from Wikipedia, see: https://en.wikipedia.org/wiki/Epoxidized_soybean_oil and also triglycerides image
9. A quick internet search identified a number of suppliers of epoxidized soybean and linseed oils. Both are sold under the Vikoflex® name, see https://www.arkema-inc.com/index.cfm?pag=16 .
10. For example, see Cytec “Radcure™ Energy Curable Resins”,
11. Figure 6 images taken from: angelescity.us, Figure 6 images;
and commons.wikimedia.org, Figure 6 images 2.
a) Soybean oil composites for structural applications such as body parts for agricultural equipment have been reported. However, such materials also contain petroleum-derived monomers. For examples, see US Patent 6,222,005, US Patent 6,890,967B2, and articles such as “Soy-Base Thermoset Plastics” at https://soynewuses.org/wp-content/uploads/44422_MOS_Plastics.pdf


Leave a Reply