In previous posts, the chemistry, and viscoelastic properties of vitrimers were presented. This post will discuss how vitrimers can be used in production to form thermoset parts/composites that can be reprocessed at end-of-life.
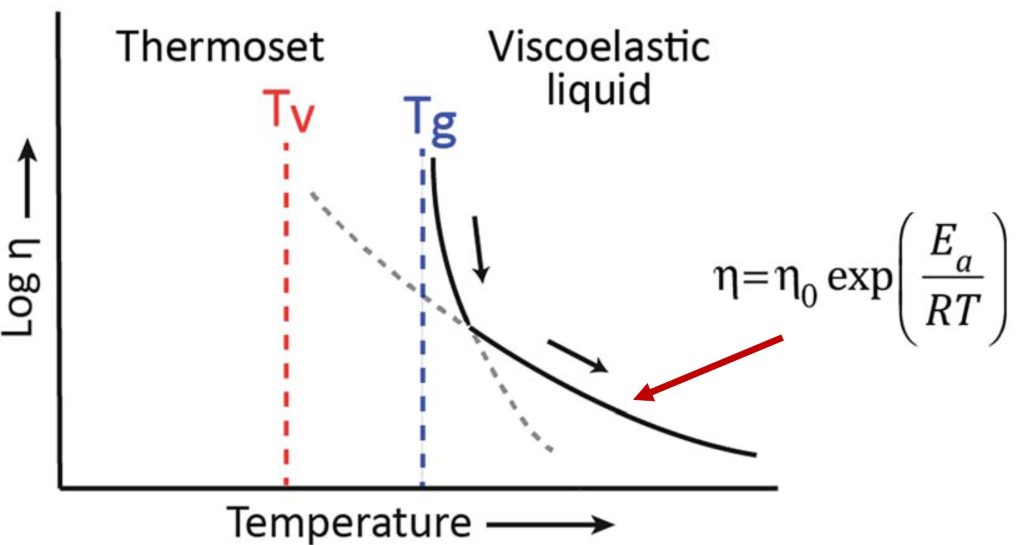
For a network that contains covalent adaptive networks, as the temperature increases, exchange reactions such as transesterification in epoxy anhydride networks can initiate. If the network is still below the Tg, the segmental mobility in the glassy state will likely suppress any topological rearrangements even though they may be kinetically activated. Upon further heating, the network will go through Tg resulting in a large drop in viscosity and increase in segmental mobility. This is shown schematically in Figure 1.
Figure 1. Tg and Tv for a hard crosslinked network [1]. Note that for a rigid thermoset, room temperature would be below Tv and Tg.
Recall that networks with CANs are fully crosslinked during curing so they will exhibit the classical viscosity/modulus decrease at Tg, as shown in Figure 1. Above Tg, the increased temperature and segmental mobility will allow for the activation of the associative covalent networks (i.e., transesterification or transamination). The viscoelasticity of the CAN networks will then follow an Arrhenius type relationship as shown above.
One potential advantage of vitrimer technology is in re-use or recycling thermoset products or composites. Mallinda is a start-up company commercializing vitrimer technology developed at the University of Colorado at Boulder. Mallinda uses a platform chemistry based on catalyst free dynamically exchangeable imine-linked polymer networks called Vitrimax [2, 3].
Image from www.mallinda.com
The thermosets are reversible (i.e., vitrimers) and may be molded after curing thus enabling re-use of scrap or flash/trim materials.
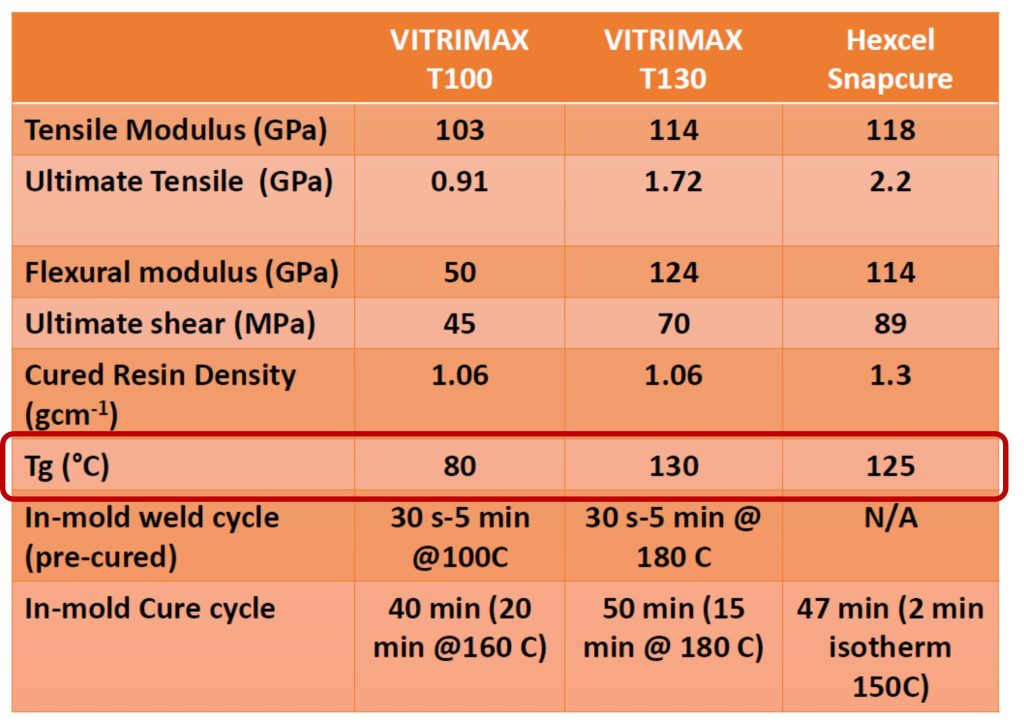
Mallinda Vitrimax resin systems are available in grades with various glass transition temperatures (Tg) and compare favorably to commercial epoxy resins as shown in Table 1.
Table 1
From the data in Table 1, the Vitrimax crosslinked vitrimer resins have similar properties to conventional thermosets and can be reprocessed after cure. In addition, Mallinda claims the cured Vitrimax resin can be depolymerized using chemical precursors to the base resin [3]. This feature allows re-use of both the fiber and the resin.
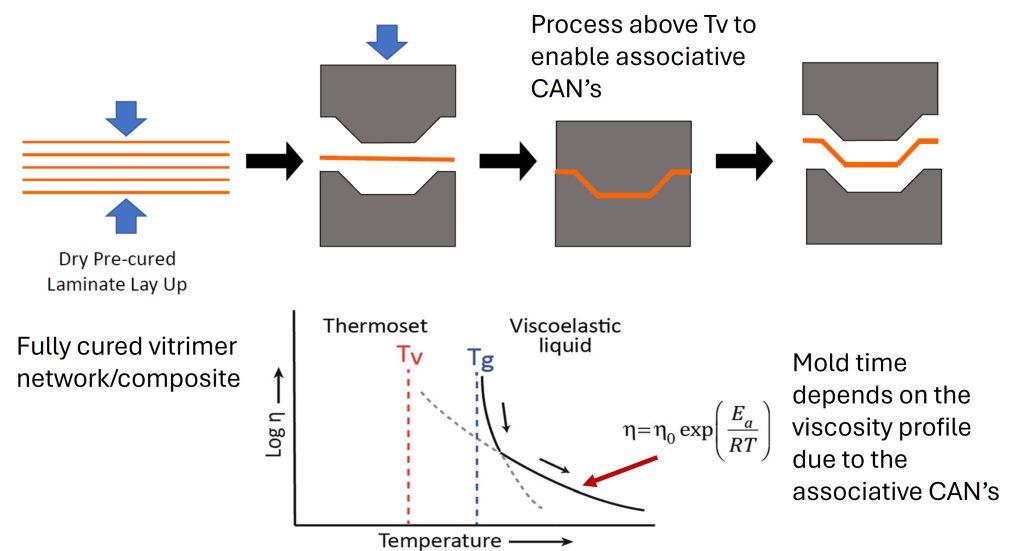
Another potential advantage for fiber reinforced composites is the ability to process after being fully cured. For example, in Figure 2 below, the starting materials do not have to be B-staged prepreg but can be precured sheets since during molding above Tv, the covalent adaptive network will allow the part to be molded. A significant advantage for this type of process is the elimination of freezer storage. Many composite prepregs must be stored in either a freezer or cold storage to preserve the shelf life (slow down the curing reaction during storage).
Figure 2. Example of vitrimer used in composite molding process [3]
Mallinda has several Vitrimax formulations with Tg’s ranging from 80-130°C and having mechanical properties (tensile modulus, ultimate tensile strength, flexural modulus, etc.) comparable to traditional commercially available prepreg/laminate systems as shown in Table 1 [3].
In summary, a vitrimer:
- Consists of an organic network of covalently bound chains with potential for high Tg’s (i.e., high crosslink density)
- May undergo thermally induced topology rearrangement via associative exchange reactions (dynamic covalent network) resulting in large changes in the viscoelastic properties at elevated temperatures.
- At high temperatures (above Tv), the viscosity is controlled by the kinetics of the associative chemical exchange reactions.
- Viscosity decrease follows the Arrhenius law.
- Typically observed in vitreous silica
- Contains permanent networks with insolubility and cross-link density is constant at all temperatures up to degradation.
References
- Denissen,W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Sci., 7, 30–38 (2016)
- Taynton, et. al., Materials, 2014, p. 1 https://doi.org/10.1002/adma.201400317
- What are Vitrimers Presentation -2021.pdf


Leave a Reply