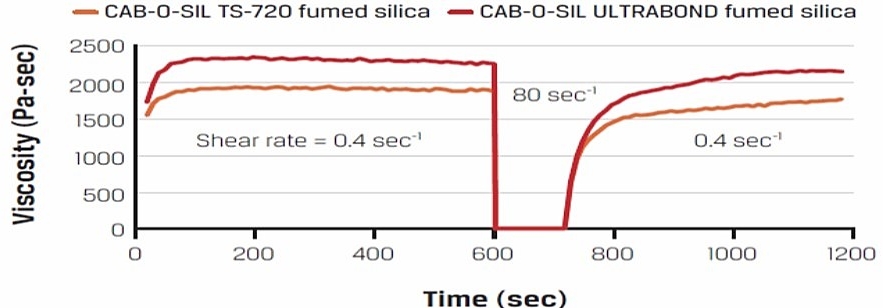
Figure above courtesy of Cabot [1]. The last post the role of fumed silica in developing thixotropy in filled thermosets. This post will expand on that discussion and provide more insights into the shear thinning and viscosity recovery process. The interesting observation in the recovery experiment is the relatively rapid recovery of structure after shearing. For the two types of Cabot fumed silica had different surface treatments. Let’s dive into looking at how the fumed silica surface treatment impacts the rheological properties.
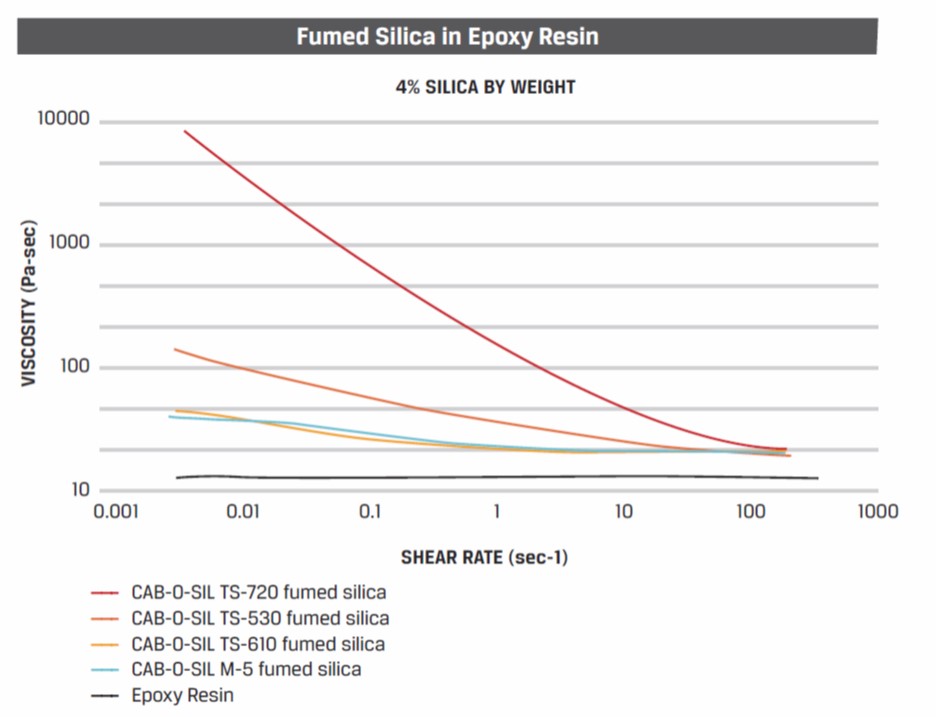
In Figure 1, the viscosity is shown as a function of shear stress for epoxy resin with 4% by weight fumed silica. Several fumed silica grades from Cabot [1] are shown.
Figure 1. Viscosity as a function of shear stress for a fumed silica filled epoxy resin.
As shown previously, the black line along the bottom is unfilled epoxy resin. As expected it has a very low viscosity and exhibits a Newtonian viscosity profile (i.e. the viscosity is independent of shear rate). The different grades of fumed silica have different surface treatments. As shown in Figure 1, the choice of surface chemistry plays an important role in tailoring the viscosity profiles. For example, the most effective fumed silica for building a yield point and high viscosity at low shear rates is TS-720 which has a polydimethylsiloxane (PDMS) surface treatment which is used in high polarity resin systems (like epoxy). A less effective fumed silica is TS-510 which has a surface treatment using hexamethyl-di-silazane (HMDZ) which is used in moderately polar resin systems. Rheological testing to obtain the viscosity-shear rate curves is an excellent analytical method to evaluate the efficacy of fumed silica in thermosetting resins.
When starting to evaluate fumed silica fillers, there are two main types; hydrophilic and hydrophobic. Hydrophilic silica does not usually function well in polar resin systems (like epoxies) since the polar resins (in this case the –OH group on the epoxy) can form large amounts of hydrogen bonding which disrupts the three dimensional network structure that gives rise to the high viscosity and development of the yield point. For epoxy resins, hydrophobic fumed silica is used to provide effective viscosity modification.
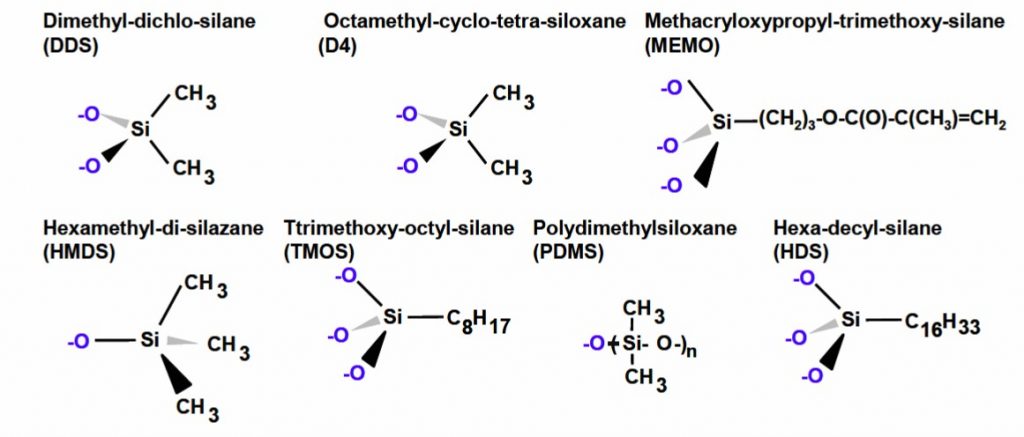
Figure 2 shows some of the surface chemistries available from Evonik Degussa [2] for the Aerosil line of fumed silica.
Figure 2. Fumed silica surface treatments and resulting surface groups (Evonik Degussa [2]).
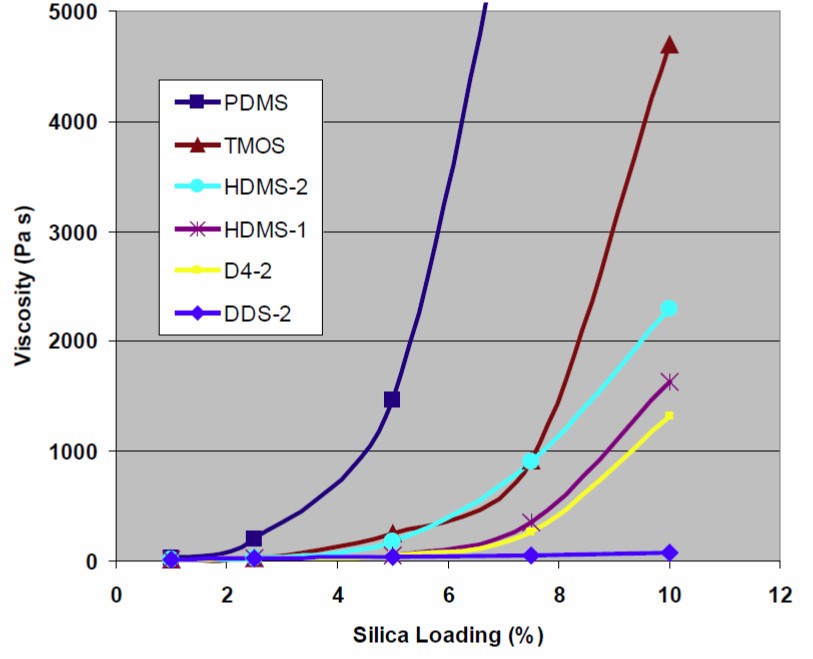
The good news for an epoxy formulator is that a wide range of surface treatments are available from the various filler suppliers. The untreated silica surface has a large concentration of surface –OH groups which can interact with the surface treatments. Figure 2 shows the resultant surface after reaction with the silica surface resulting in the –O-Si bridges to the functional groups. In the paper by Toth, et. al [2] from Evonik Degussa, they evaluated impact of several surface treated fumed silicas on the rheological properties. Several different treated fumed silica fillers were added to EPON® 828 (DGEBA type epoxy resin from Hexion) at loading levels from 1-10 weight percent and mixed using a dual-axis centrifugal mixer. After mixing, the epoxy-fumed silica samples were allowed to equilibrate at room temperature for at least 24 hours prior to rheological testing. Since the fumed silica mixtures are sensitive to both time and shear history, it is very important to carefully control the sample prep and shear history prior to rheological testing. The rheological properties were measured using a cone and plate Haake RX6000 rheometer and the viscosity at a shear rate of 0.1 sec-1 was recorded. The viscosity data for the various filler loadings and surface treatments is shown in Figure 3.
Figure 3. Viscosity as a function of fumed silica loading in EPON 828 epoxy resin [2].
The viscosity data in Figure 3 shows clearly demonstrates that the surface treatment has a dramatic impact on the thickening efficiency in epoxy resins. The fumed silica with the PDMS surface treatment provides the most effective thickening as a result of the high degree of hydrophobicity and the long polydimethylsiloxane chains. The PDMS chains interact with the siloxane chains on adjacent particles allowing the build of the three-dimensional fumed silica network. As discussed previously, the particle-particle interactions in the three-dimensional network cause a large increase in the viscosity at low shear rates. The next most efficient thickener is trimethoxy-octyl-silane (TMOS) with an eight carbon hydrophobic chain extending from the silica surface (see Figure 2). Several other surface treatments provide lesser thickening efficiency. In some cases, you would want high filler loadings such as to reduce the coefficient of thermal expansion, but desire low thickening. In this case, the short chain dimethyl-dichloro-silane (DDS) provides the least amount of viscosity modification. Note: it appears that that in Figure 3, the labels in the legend are incorrect for the HMDS surface treatments. HDMS-2 and HDMS-1 are actually hexamethyl-di-silazane (HMDS or sometimes referred to as HMDZ).
The surface treatments will not only impact the thickening efficiency but also control the rate of recovery. Note that in Figure 3, the PDMS exhibited the largest viscosity modification for a given filler loading. Additionally, the data from Cabot in the top figure, the TS-720 with the PDMS surface treatment showed a rapid structure recovery after steady shear at high shear rates. The choice of surface modification allows the formulator to “dial in” or tailor the rheological properties for the specific dispensing or end use application.
References:
- Cabot datasheet for CAB-O-SIL TS-720
- James Toth, Rodney Conn, and Anthony Gosselin, Evonik Degussa Corp. Thermoset Resin Formulators Association in San Antonio, Texas September 13 – 14, 2010

Leave a Reply