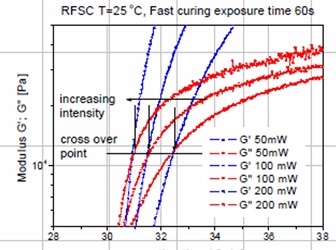
 In the previous post the role of UV intensity at a constant exposure time was shown to have a large impact on the curing kinetics. As the UV intensity increased, the time to gelation decreased from the faster chemical crosslinking in an acrylate pressure sensitive adhesive. Additionally, the UV rheometer data also showed that the modulus at the gel point was approximately the same for three UV exposure intensities another indication that gelation for this type of UV curing mechanism is an iso-conversion point. This can be seen in the figure on the left.
In the previous post the role of UV intensity at a constant exposure time was shown to have a large impact on the curing kinetics. As the UV intensity increased, the time to gelation decreased from the faster chemical crosslinking in an acrylate pressure sensitive adhesive. Additionally, the UV rheometer data also showed that the modulus at the gel point was approximately the same for three UV exposure intensities another indication that gelation for this type of UV curing mechanism is an iso-conversion point. This can be seen in the figure on the left.
In practical applications, it is important to determine the correct UV dosage.
UV dosage = I X t
- I is the UV intensity (mW)
- t is the exposure time (seconds)
To investigate the impact of UV intensity as a function of time, a series of experiments were run at constant UV intensity of 30mW with the exposure time varied from 30 seconds to 240 seconds. Figure 1 shows how the dynamic storage and loss moduli (G’ and G” respectively) change as a function of time.
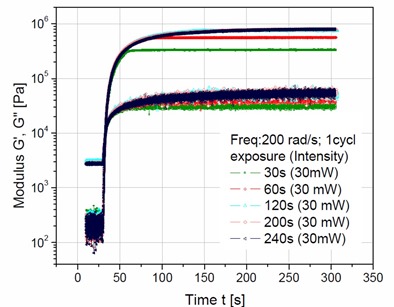 Figure Courtesy of TA Instruments
Figure Courtesy of TA Instruments
Figure 1. Dynamic Moduli as a function of time for various exposure times at a constant 30mW intensity.
The UV exposure causes a rapid increase in both the storage and loss moduli indicating rapid crosslinking due to generation of free radicals by the photoinitiators. After 30 seconds of UV exposure, the storage modulus, G’ reaches a plateau of approximately 3 x 106 Pa (green curve in Figure 1). This corresponds to a UV dosage of 900 mJ (0.9 Joules). With a 60 second exposure time (1.8 Joules), the storage modulus, G’ reaches a plateau of approximately 5.5 x 106 Pa (red curve in Figure 1). At exposure times less than 60 seconds, there is not enough UV dosage to fully cure the acrylate PSA evaluated here. Increasing the exposure to 120 seconds or longer yields approximately the same storage modulus after 200 seconds of reaction time. In Figure 1, note that all of the storage moduli curves reach the same value (dark blue top curve in Figure 1) for exposure times of greater than or equal to 120 seconds. Inspection of the data in Figure 1 indicates it would is difficult to determine the exact role of UV dose on the final storage moduli. In order to evaluate the impact of UV dose the dynamic storage moduli, G’ at full cure (the asymptotic values in Figure 1) was plotted as a function of UV dosage in Joules in the Figure 2.
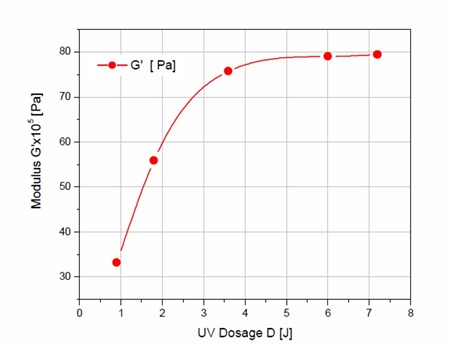 Figure Courtesy of TA Instruments
Figure Courtesy of TA Instruments
Figure 2. Final dynamic storage modulus (G’) as a function of UV dosage in Joules
As can be observed in Figure 2, the UV dose plays a strong role in developing the fully cured storage modulus. The UV dose represents the total amount of energy applied to the sample. In Figure 2, the storage moduli for each UV dose continues to increase with UV dose until a minimum of 6 Joules is applied. The UV rheometer experiments indicated for the acrylate PSA in this example, a UV dose of at least 6 Joules would be required to achieve a fully cured adhesive.
In the last four posts we have demonstrated the utility of using a specially modified rheometer to follow the rheological changes during UV exposure. There are several commercially available instruments with UV parallel plate geometries for these types of experiments. In the next series of posts we will explore the use of UV Differential Scanning Calorimetry (UV-DSC) to investigate the curing kinetics of UV cured polymers.

Leave a Reply