Guest Post by Dr. R. Bruce Prime
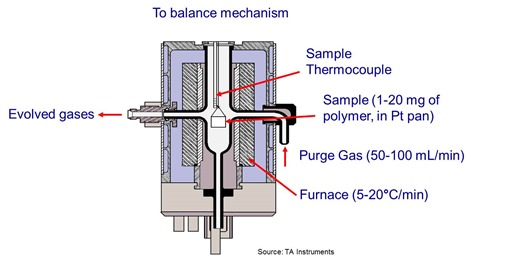
Thermogravimetric analysis or TGA is a technique where the mass (or weight) of a material is measured as a function of temperature or time while the sample is subjected to a controlled temperature program in a controlled atmosphere [Earnest, ASTM STP 997 (1988)].
Temperature ranges for commercial TGAs are typically ambient to 1000°C or higher, more than sufficient for thermoset applications. As shown in the illustration a purge gas flowing through the balance creates an atmosphere that can be inert such as nitrogen or oxidizing such as air or oxygen. Additionally, the moisture content of the purge gas can be controlled anywhere between dry and saturated. Typical sample size ranges from less than a milligram to a gram or more.
Thermosets generally exhibit mass loss, which falls into three categories: volatile components, reaction products, and decomposition products. All of these mass loss processes may be characterized by TGA to yield information such as moisture content, residual solvent, composition, extent of cure and thermal stability. The kinetics of these processes may also be determined to model and predict cure and aging due to thermal and thermooxidative processes. Kinetics will be the subject of future posts.
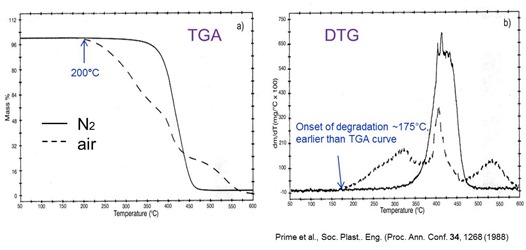
The TGA plots above show the degradation of a polymer when heated at 10°C/min and illustrate several attributes of TGA. The polymer is poly(vinyl methyl ether) or PVME, a key component of a thermosetting disk coating [Prime et al., Soc. Plast. Eng. Proc. Ann. Conf. 34, 1268 (1988)]. Notice that the degradation in nitrogen is simple with one major mass loss step that begins above 300°C, whereas in air three mass loss processes are observed with the first beginning around 200°C. Advantages of the derivative d(mass)/dt or DTG curves are also evident. The three mass loss processes in an oxidizing environment are much easier to distinguish. And the enhanced sensitivity of the DTG curve is illustrated in the 25°C earlier detection of the onset of degradation in air than is observed in the TGA curve.
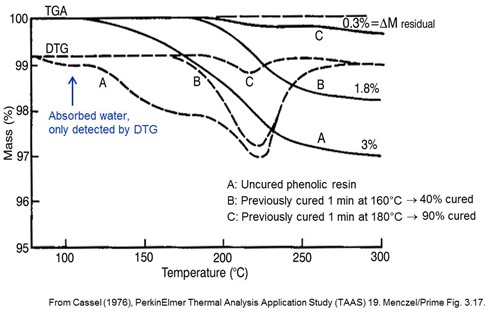
This set of TGA curves for a phenolic resin illustrates the application of TGA to the measurement of cure for thermosets whose cure chemistry involves a distinct mass loss (e.g. water or formaldehyde) for each bond that is formed. The uncured resin shows a very small mass loss due to absorbed water that is only observed in the DTG curve plus a two-stage cure process with a total mass loss of 3%. Data on the two partially cured samples offer valuable insight into the cure process plus an estimate of the degree of cure. Note that cure of this material could not be measured by DSC because the cure exotherm would be altered or masked by the endothermic evaporation of the reaction products.
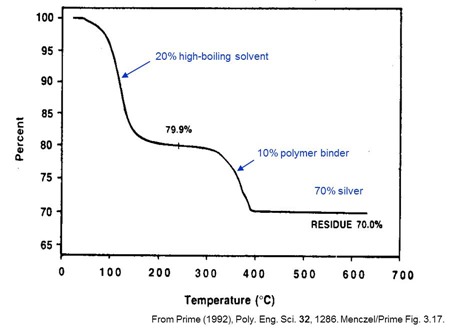
The example above clearly shows the composition of a silver-filled thermosetting ink from the two distinct mass loss steps. This is typical of an ink used in screen-printing. TGA was run at 5°C/min in air.
To summarize, TGA of thermosets yields valuable information on:
- Volatile components: absorbed moisture, residual solvents, additives/oligomers from ambient to 200°C
- Reaction products, e.g. from cure of phenolic resins, blocked catalysts from 100 to 250°C
- Volatile degradation products from 250 to 800°C
In the next post we will explore more applications of TGA to thermosets.

Leave a Reply