Guest Post by Dr. Robert Humphreys
 Depending on your age, chances are your parents, grandparents or great-grandparents made most of their telephone calls with a rotary-dial phone manufactured with Bakelite. It was heavy, came in black only, and was almost indestructible. If you have children or grandchildren under the age of 20, show them the image. Unless they watch “Leave it to Beaver” reruns with Grandma, they probably will wonder how people sent text messages or Tweeted with such a device.
Depending on your age, chances are your parents, grandparents or great-grandparents made most of their telephone calls with a rotary-dial phone manufactured with Bakelite. It was heavy, came in black only, and was almost indestructible. If you have children or grandchildren under the age of 20, show them the image. Unless they watch “Leave it to Beaver” reruns with Grandma, they probably will wonder how people sent text messages or Tweeted with such a device.
Bakelite is made from a formulated thermoset phenolic resin and was the first commercial plastic 1. Phenolic resins remain commercially important today because they are low cost, straightforward to manufacture, can be formulated for a broad range of applications, and, once cured, exhibit exceptional durability in applications requiring prolonged exposure to moisture, acids, cleaning solutions, lubricants and elevated temperature. Markets for phenolic resins include: construction adhesives for wood (plywood, oriented strand board, particle board); molding compounds for laminates, friction materials such as brake pads and clutch pads, abrasive products such as grinding wheels, foundry molds and rigid parts such as gears, pulleys and rollers; impregnation of paper and cloth for engine filters; binders for printing inks and tire manufacture; and even photoresists 1,2.
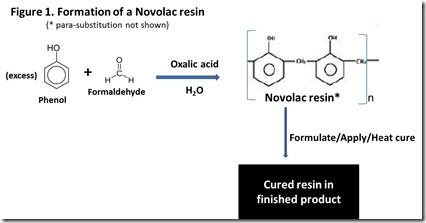
In their simplest form, phenolic resins are products from reaction of phenol or a substituted phenol with formaldehyde. Resins fall into two basic classes, depending on ratio of phenol to formaldehyde and process chemistry. Novolacs result from acid-catalyzed condensation of formaldehyde with phenol at less than a 1-to-1 molar ratio, thus favoring reaction of formaldehyde with multiple phenols. The result is a non-cross-linked, more or less linear oligomer of aromatic rings connected by methylene groups, as illustrated in Figure 1 3 (note that both ortho- and para-substitution will occur on the phenol ring, but only ortho-substitution is shown). Because of the deficiency of formaldehyde relative to phenol, Novolacs have no reactive functionality remaining and will not cure to form cross-linked materials until additional formaldehyde is added and heat is applied. Additional formaldehyde is usually added as hexamethylene tetramine or paraformaldehyde 4.
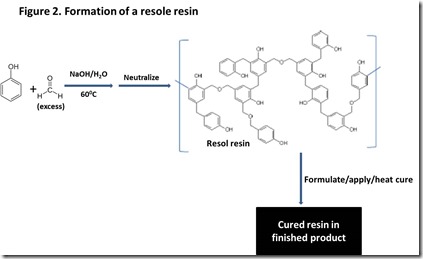
The other major class of phenol formaldehyde resins is resols. Resols are prepared under basic conditions with a formaldehyde-to-phenol ratio greater than one (Figure 23). This ratio results in more than one hydroxymethyl group on some phenol rings, so the resin will eventually cross-link sufficiently such that it will become insoluble in the reaction medium. To avoid reaching the gel point, the resin viscosity is monitored and heating is stopped when the viscosity reaches a target value and the mixture is neutralized. The excess of hydroxymethyl groups means that the resol will cross-link upon further heating without the need to add additional formaldehyde.
Most phenolic resins are made with phenol or a few substituted phenols, such as alkylated phenols like cresol and hydroxyphenols like resorcinol. These compounds are produced primarily from petroleum, although coal tar has been a major source in the past. Formaldehyde, the other key reactant, is produced by catalytic oxidation of methanol, which in turn is generated from natural gas via steam reforming to synthesis gas. Synthesis gas can also be produced by gasification of biomass, providing a route to renewable methanol (equation 1)5. So, the critical question for production of renewable phenolic resins is, how might renewable phenol and substituted phenols be produced?
Equation 1: Natural gas or coal or biomass → 4 H2 + 2 CO → 2CH3OH
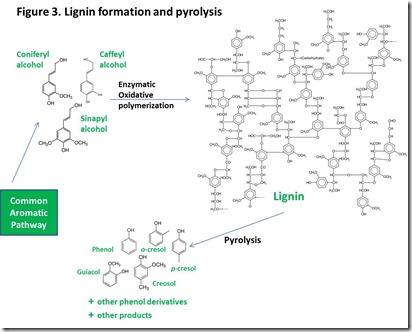
Nature is very good at making aromatic molecules and polymers, including many that can be described as substituted phenols. Lignin, second only to cellulose/hemicellulose as the largest volume biological material produced by Nature6, is assembled by oxidative coupling of three major aromatic molecules that can be viewed as substituted phenols: caffeyl alcohol, coniferyl alcohol and sinapyl alcohol (Figure 33). It has been demonstrated that controlled pyrolysis of lignin isolated from a number of plant sources can yield significant amounts of a number of phenol derivatives7 that are important in production of phenolic resins, as illustrated in Figure 33. Commercial production of non-food biomass-derived sugars at the scale needed to serve the bio-fuels and renewable chemicals industries will generate an enormous amount of lignin. The best current option for utilizing this lignin is burning for its fuel value, so higher-value options for utilizing this renewable material, such as production of phenolic chemicals, would be welcomed by the industry.
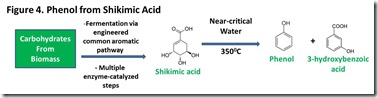
The major metabolic pathway that Nature employs to produce aromatic compounds such as coniferyl, caffeyl, and sinapyl alcohols, as well as the aromatic amino acids tyrosine, phenylalanine and tryptophan, is known as the common aromatic pathway8. Shikimic acid is a key metabolite that is generated along this pathway. The common aromatic pathway in organisms such as E.coli can be engineered to enhance shikimic acid production. It has been shown that reaction of shikimic acid in water close to the critical point can produce phenol in over 50% yield8 (Figure 43). This pathway is already used commercially by the pharmaceutical industry to produce shikimic acid.
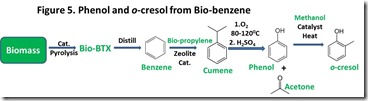
A third route to renewable phenol utilizes bio-benzene produced by pyrolysis of biomass or sugars derived from biomass. Catalytic pyrolysis of biomass to produce bio-BTX (benzene/toluene/xylenes) was described in several previous posts on this site9. Bio-benzene isolated from the bio-BTX mixture could be substituted directly in the current petrochemical process for making important phenolic resin raw materials, including phenol and substituted phenols10 (see Figure 53). This route has the potential to produce a broad range of renewable fuel and chemical components, mimicking the way petroleum is used.
As with most large-volume, commercially important polymers, it is unclear which technology will be the winner for production of renewable phenolic resins. Nevertheless, with an annual global volume of over 4 million tonnes (2006 data11), replacing this century old petroleum-based polymer technology with renewable equivalent materials will make a significant contribution to the drive to a more sustainable economy. As we will see in the next post in this series, Novolacs also will be key components of renewable epoxy resin technology.
References:
1. “Phenolic Resins”, available at https://wpage.unina.it/avitabil/testi/PheForm.pdf
2. a) “High performance thermoset composites”, Norplex Micarta, available at reference 2a link ; b) “Basf expands and modernizes phenolic resin production at Ludwigshafen”, available at https://www.worldofchemicals.com/media/basf-expands-modernizes-phenolic-resin-facility-in-ludwigshafen/6048.html .
3. Molecular structure images for resole resin), and www.bmrb.wisc.edu (coniferyl and sinapyl alcohol), commons.wikimedia.org (lignin), all other structure images from Wikipedia.
4. a) A. Pizzi, “Phenolic resin adhesives”, Chapter 26 in Handbook of Adhesive Technology, A. Pizzi and K.L.Mittal editors, CRC Press, 2003 ; b) Kenneth Bourier, “Phenolic Resins” in Coatings Technology Handbook, 3rd edition, Arthur A. Tracton, editor, CRC Press, 2005.
5. a) A.E.Hokanson and R.M.Rowell, “Methanol from wood waste: a technical and economic study”, June 1977, Forest Products Laboratory, available at https://www.fpl.fs.fed.us/documnts/fplgtr/fplgtr12.pdf ; b)George W. Huber, Sara Iborra, and Avelino Corma, “Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering”, 2006, Chemical Reviews, 106, 4044-4098. See sections 3.1 and 4.2.
6. Lignin overview, Institute of Paper Science and Technology, Georgia Tech, available at reference 6 link .
7. Venugopal Mendu, Anne E. Harman-Ware, ark Crocker, Jungho Jae, Jozsef Stork, Samuel Morton, Andrew Placido, George Huber and Seth DeBolt, “Identification and thermochemical analysis of high-lignin feedstocks for biofuels and biochemical production”, Biotechnology for Biofuels, 2011, 4: 43, available at https://www.biotechnologyforbiofuels.com/content/4/1/43 .
8. See Figure 8.18 and page 212 in David Dodds and Bob Humphreys, “Production of aromatic chemicals from biobased feedstock”, Chapter 8 in Catalytic Process Development for Renewable Materials, eds. Pieter Imhoff and Jan Cornelis van der Waal, Wiley-VCH, Germany, 2013, and references cited.
9. See “Feeding the bio-refinery 3: Conversion of biomass to basic chemicals”, August 3, 2012, biorefinery 3 blog link ; “The winding road to renewable thermoset polymers: Part 2 Unsaturated Polyester (UP) Resins”, July 8, 2013, https://polymerinnovationblog.com/ .
10. Manfred Weber and Markeu Weber, “Phenols”, Chapter 2 in Phenolic Resins: A Century of Progress, L. Pilato, editor, Springer-Verlag, Berlin, 2010, available at reference 10 link .
11. “2006-2007 World Phenolic Resin and Plastic Industry Progress”, available at reference 11 link .

Hi I wanted to know what was the conversion rate of phenol when producing the Novolac resin under the Oxalic catalyst.