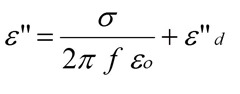 In the previous post we discussed the importance of the ionic conductivity as a useful probe of thermoset cure. In the equation on the left, one observes that the dielectric loss factor, e” is proportional to the ionic conductivity, s. The term e”d represents the combined (or lumped) contributions from all dipoles in the curing system. This relationship forms the basis for using dielectric spectroscopy to investigate thermoset curing.
In the previous post we discussed the importance of the ionic conductivity as a useful probe of thermoset cure. In the equation on the left, one observes that the dielectric loss factor, e” is proportional to the ionic conductivity, s. The term e”d represents the combined (or lumped) contributions from all dipoles in the curing system. This relationship forms the basis for using dielectric spectroscopy to investigate thermoset curing.
Remember, most curing thermosets contain some amount of ionic impurities which act as “atomic level” probes to the segmental mobility during curing. As the curing thermoset chain extends, the viscosity increases resulting in a decrease in the mobility of chain segments. The small ions are moving in the electric field and thus provide a convenient means to prove curing. As the curing thermoset passes the gel point, the viscosity increases very rapidly due to the formation of a tight network structure. An additional very useful feature is the ions probe local environments and can monitor the curing through gelation and vitrification. This will prove quite useful as an in-situ cure monitor.
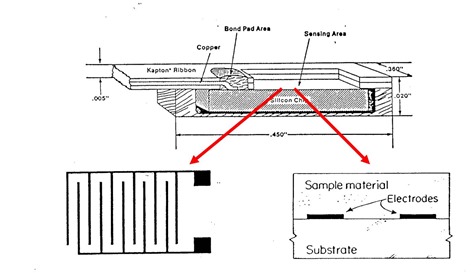
Let’s dig into some of the details of how to make dielectric measurements. In the work presented here, a Micromet Microdieletric measurement system was used in the inter-digitated comb electrode configuration.
Source: Micromet Instruments, now part of Netzsch Instruments (1)
In the above figure, the dielectric sensor is shown in the top figure. The sensors come as an integral package with the sensing area consisting of inter-digitated comb electrodes. In the schematic on the right, a cross-section shows how the sample material covers the electrodes. This feature allows the sensors to be embedded in various type of process tools, since during curing, the materials will soften, flow over and cover the electrodes allowing dielectric measurements.
First, let’s examine the isothermal during process. We will use the work of Prof. Sue Ann Bidstrup at Georgia Institute of Technology as a nice example (2). They examined the isothermal curing of DGEBA and DDS:
- DGEBA (diglycidyl ether of bisphenol-A, a common difunctional epoxy)
- DDS (diamino diphenyl sulphone, a common hardener for aerospace epoxies)
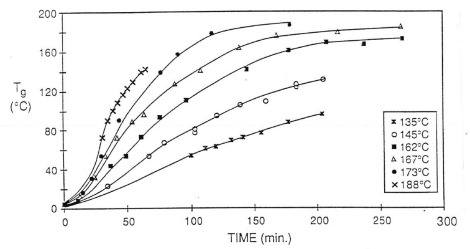
To establish the curing relationship, they measured the glass transition temperature (Tg) as a function of cure time at various isothermal temperatures. The Tg-time data is plotted in the following figure:
As we have shown in previous posts, the curing rate is strongly dependent on the isothermal cure temperature. The Tg-time relationship establishes the baseline for dielectric measurements.
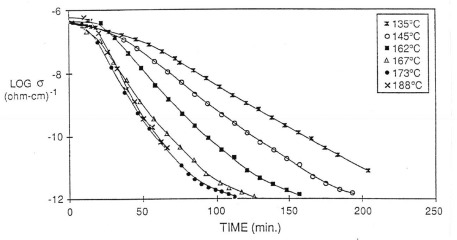
To investigate how the dielectric response correlates to the changes in Tg, the dielectric loss factor was measured for the DGEBA/DDS resin at the same isothermal temperatures in the figure above. In the following figure, the log of the ionic conductivity is plotted as a function of time at various isothermal temperatures:
From the data above, the ionic conductivity provides a very useful probe for thermoset curing. Remember the ionic conductivity is a probe of segmental mobility, so as the epoxy system above crosslinks, the segmental mobility steadily decreases (analogously, the Tg increases). The dielectric data clearly shows how the curing rate increases with temperature (the slope of the ionic conductivity/time plot gets steeper as the temperature increases).
We now have a basis for using dielectric methods to probe curing during processing. In the next post we will discuss dielectric cure monitoring during non-isothermal curing.
References:
1) Netzsch Instruments, https://www.netzsch-thermal-analysis.com/us/products-solutions.html
2) Sue Ann Bidstrup, Norman Sheppard, and Stephen Senturia, Polymer Engineering and Science, Vol. 29, p. 325-328, 1989

Leave a Reply