 In the last post we discussed the background work that led to my interest in evaluating if the dielectric response during curing was related to the rheological response. We showed that the equations for both measurements were similar. In this post we will highlight some important differences in the two measurements and show how the similarities can also be exploited.
In the last post we discussed the background work that led to my interest in evaluating if the dielectric response during curing was related to the rheological response. We showed that the equations for both measurements were similar. In this post we will highlight some important differences in the two measurements and show how the similarities can also be exploited.
First, lets get some definitions on the table. The dielectric terminology can be a bit confusing so we will take time here to set the stage for future posts.
Dielectric Permittivity:
- represents the polarization of the medium
- typically called the “dielectric constant” but for curing systems the dielectric “constant” changes as a function of the cure state and temperature
Dielectric Loss Factor arises from two sources:
- Energy loss associated with time-dependent dipolar relaxations
- Bulk (or ionic) conduction
For most curing thermosets, for example epoxies, they have polar groups that give rise to dipoles that can respond to the applied voltage. A good example would be the hydroxyl groups formed during amine-epoxy curing. Additionally, most thermosets are not completely pure, that is they have some residual ions from the synthetic process used to make them. In epoxies, there is residual chlorine and sodium from the process used to put the epoxy functionality on bisphenol A. As we will see, these residual ions (even at low levels) can be very useful probes for the dielectric measurements.
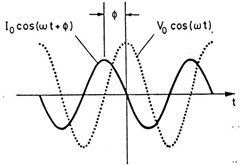
For cure monitoring, we are going to focus on the dielectric loss factor. The dielectric loss factor is given by:
From the above relationship, one observes the dielectric loss factor is comprised of two components; one from ionic conductivity and one from the contributions from the dipoles. The main differences between the dynamic mechanical measurement is that the dielectric loss factor has a component that arises from the ionic conductivity (i.e. from the movement of the chlorine or sodium impurities) which we will see is a key factor in probing the curing of thermosets.
Let’s take a closer look at the components of the ionic conductivity:
The strength of the ionic conductivity comes from three factors
- the charge magnitude (that is how “strong” is the chlorine or sodium) of the ionic species
- the amount of the species
- and lastly, but most important, is the ion mobility. This is the key feature allowing the dielectric measurements to probe curing.
Let’s do a little mental gymnastics to understand the role of ion mobility in cure monitoring. Let’s think about a chlorine or sodium ion that is in a sea of monomers and oligomers (such as in a prepreg). Since the thermoset isn’t fully cured (could be partially cured as in the case of the prepreg) so during heating, the viscosity will decrease, or for prepregs, on heating, the resin will go through the Tg resulting in a much lower viscosity and higher segmental mobility. Probing the dielectric loss factor in the glassy state would result in a very low ion mobility. As temperature increases, the short chains, unreacted monomers and oligomers can have greater segmental mobility. That is, it will be easier for those embedded ions to move in response to the applied voltage.
Remember from the ionic conductivity equation above, the ions have a charge and will move in response to the applied voltage. Voila, we now have a really nice probe of how the movement of the molecular segments changes with curing. As the thermoset cures, the first chemical reaction is chain extension (i.e. amine nucleophilic addition to the epoxy group) and then as the degree of polymerization increases, crosslinking occurs. As the molecular weight increases, the mobility of the chain segments decreases. Once the crosslinking reaction kicks in, the segmental mobility will decrease very quickly.
In subsequent posts we will provide examples of how to use the dielectric loss factor to monitor curing during isothermal and non-isothermal temperature profiles.


Hi Jeff,
I am one of the co-founders of Micromet Instruments, which you mentioned in Part 1 of your series about dielectric cure monitoring. By coincidence I just ran across your blog and began reading it with great interest. In 2009 I and another engineer from the old Micromet days started a new company dedicated to dielectric cure monitoring and continuing where Micromet Instruments left off. It is Lambient Technologies and our web site is:
http://www.lambient.com.
We have many application notes dealing with both theory and practice, and all are welcome to visit the site and download them. We have also released for free download “The Handbook of Dielectric Analysis and Cure Monitoring,” which contains 150 pages of information derived from our 30 years of experience in the field.
I remember you from many years ago, when you worked with our Microdielectrometer System III, and want to say “Hello.”
Huan Lee
Lambient Technologies
e-mail: info@lambient.com