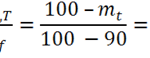Happy New Year and Best Wishes for a Prosperous 2015 I read an very interesting article over the weekend in Fortune Magazine (January 2015) titled “Start-up: You.” The premise of the article is that employment as we have known it had fundamentally changed. Additionally, the way on progresses in your career is also different. Gone are the days of corporations guiding you ... [Click to Continue...]
Happy Holidays
From all of us at Polymer Innovation Blog, we wish you and your families a safe and relaxing holiday season. We will be back in 2015 with some exciting new topics. Dr. R. Bruce Prime and Dr. Larry Judovits will have a multiple part series on Modulated Temperature DSC coming up in mid-January. I will be back with more thermoset rheology including dielectric ... [Click to Continue...]
Thermoset Cure Kinetics Part 8: DMA Kinetics
Guest Post by Dr. R. Bruce Prime In this post we describe how DMA can contribute to the study of cure kinetics. We will concentrate on areas where DSC fails to yield useful kinetic data or where Tg is the preferred or even the only way to get a handle on the kinetics. One example is phenolic based thermosets described in the last post, where the endothermic mass loss ... [Click to Continue...]
Thermoset Cure Kinetics Part 7: TGA Kinetics
Guest Post by Dr. R. Bruce Prime In the previous posts in this series we introduced basic principles of kinetics and the application of DSC to measure cure kinetics. Here we describe how thermogravimetric analysis or TGA can be applied to thermoset cure when the loss of mass is an integral part of the cure chemistry. The most common example is phenolics, where each bond ... [Click to Continue...]
Thermoset Cure Kinetics Part 6: A Practical Example of Cure Kinetics in Action
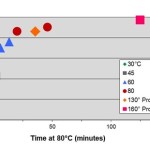
Guest Post by Dr. R. Bruce Prime In Parts 1 - 5 of this series we have described basic kinetic principles, how to measure activation energy E, the role of isothermal DSC in kinetic analyses, and time-temperature superposition kinetics. Each of those may be viewed as a tool in our kinetics tool box. Here we describe how they were all used to solve an actual problem involving a ... [Click to Continue...]