This is the final post in this series on high performance thermosetting polymers. This post discussed the use of specialized cyanate esters for high performance electronic packaging applications.
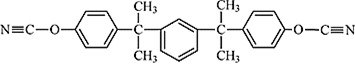
Dicyanate resins are versatile thermosets and have been used in a wide variety of applications. For example, RTX-366 (Hunstman Chemical), shown in Figure 1, is a high molecular weight meta substituted cyanate ester oligomer with excellent dielectric properties (low dielectric constant and loss factor) along with low moisture absorption [1,2]. RTX 366 has been used in low outgassing and dimensionally stable, high tolerance composites for communication satellites, space antennas, microwave transparent radomes, and aircraft film adhesives.
Figure 1. Meta substituted multi aromatic dicyanate (Huntsman RTX-366)
One of the major drawbacks of cured cyanate ester networks is low fracture toughness (due to high Tg and highly aromatic network structure) and high moisture absorption (thought to be due to the large amount of free volume between the triazine rings).
Work at IBM in the early 1990’s by Gotro and coworkers [3] investigated using RTX366 (the meta substituted dicyanate shown in Figure 1) to achieve low dielectric constant and low loss factor composites with lower moisture absorption and improved fracture toughness.
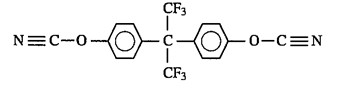
The work focused on evaluating blends of M-dicyanate monomers shown in Figure 1 with a fluorinated bisphenol dicyanate shown in Figure 2.
Figure 2. Fluorinated bisphenol dicyanate
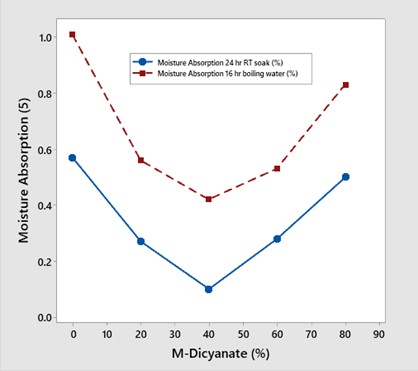
A series of blends were formulated with M-dicyanate concentration from 0-80 wt%. The moisture absorption was measured at two conditions 1) 24-hour soak in room temperature water and 2) 16 hours immersion in boiling water. The data is shown in Figure 3.
Figure 3. Moisture absorption versus meta-dicyanate content at two test conditions noted in the figure [3].
The formulation strategy was to use the meta link in the tri-aromatic monomer to disrupt the triazine ring structure to achieve the objectives of lowering the moisture content and improving the fracture toughness. At concentrations up to 40 wt% M-dicyanate, the moisture absorption decreased. Surprisingly, there was a minimum at 40 wt% and a subsequent increase in the moisture absorption at 60 and 80 wt% M-dicyanate.
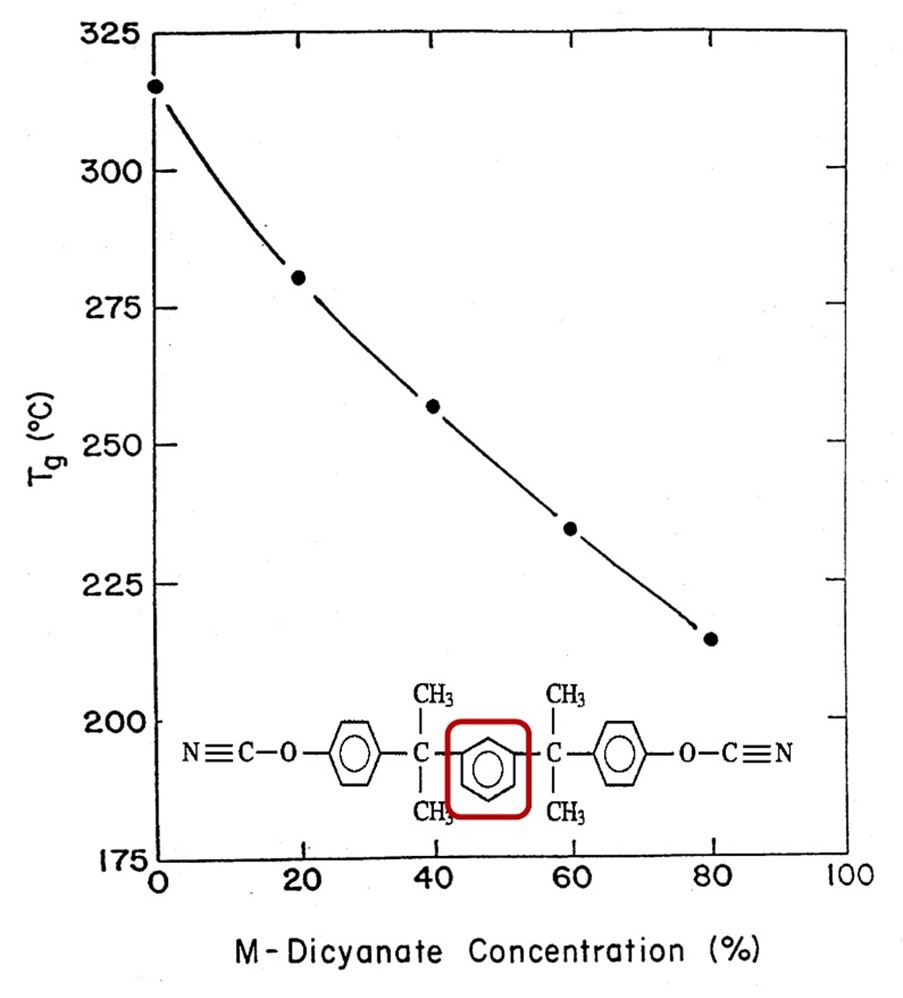
The next experiment was to measure the glass transition temperature, Tg as a function of M-dicyanate concentration using differential scanning calorimetry (DSC). The Tg is plotted as a function of M-dicyanate concentration in Figure 4.
Figure 4. Glass transition temperature (Tg) versus meta-dicyanate content [3].
In Figure 4, the Tg decreases monotonically as a function of the M-dicyanate concentration as expected. Note there is a steady decrease in Tg and no discontinuity is observed in the vicinity of 40 wt % M-dicyanate. The data shows the increase in flexibility imparted by the meta linkage (shown in the red box in Figure 4) in the M-dicyanate caused a reduction in the Tg. In the work, a lower Tg was acceptable since the main formulation goals were to decrease moisture absorption and increase the fracture toughness while maintaining the dielectric constant of 2.6 – 2.8.
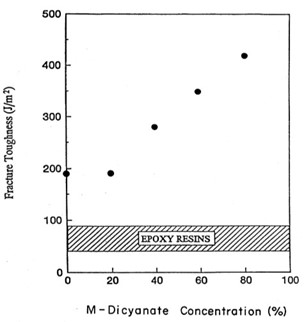
The next step in the testing was to evaluate the fracture toughness as a function of the M-dicyanate concentration. The fracture toughness was measured on a center notched beam loaded in three-point bending per ASTM D5045 [4]. Figure 5 shows the fracture toughness or G1C (the strain energy release rate in Mode 1) as a function of the M-dicyanate concentration.
Figure 5. Fracture toughness (or strain energy release rate G1c in J/m2) versus meta-dicyanate content [11].
In Figure 5, at loadings of 20 wt % M-dicyanate, the fracture toughness does not increase. Note that above 20 wt% there is a linear increase in the fracture toughness with no discontinuity noted at 40 wt%. Figure 8 also shows a comparison for epoxy resins. The band represents typical fracture toughness values for epoxy resins (no blending or mixtures).
We have seen that the Tg and the fracture toughness exhibit the expected decrease in Tg and increase in fracture toughness with the introduction of the multi-aromatic with flexible linkages. The investigators postulated that the meta link in the RTX 366 caused a reduction in free volume by occupying the “open spaces” in the triazine network. Packing density or orientation effects in the narrow range in the vicinity of 40-wt% caused the moisture absorption to decrease. At higher M-dicyanate concentrations, the moisture absorption again increased.
References
- Shimp and W. Craig, 34th Int. SAMPE Symp. 222 (1989).
- Shimp and S. Ising, 34th Int. SAMPE Symp. 1045, (1989).
- US Patent 5,527,838, Gotro et. al.
- ASTM D5045 Standard Test Methods for Plane-Strain Fracture Toughness and Strain Energy Release Rate of Plastic Materials